The systemic toxicity introduced into the oral environment by tobacco smoke is profoundly complex, extending far beyond superficial staining or simple halitosis; it initiates a cascade of destructive biological processes that fundamentally compromise the resilience and architecture of the gum tissues and their underlying support structures. To classify the damage merely as ‘gingivitis’ or ‘periodontitis’ is to miss the intricate, cellular sabotage orchestrated by the thousands of chemical constituents within cigarette smoke. This is not just an irritant; it is a systemic antagonist that modifies microcirculation, disrupts the finely tuned immune response, and shifts the ecological balance of the oral microbiome into a more hostile state. The resulting periodontal pathology in a smoker is often deceptively subtle in its early clinical presentation, yet relentlessly aggressive in its progression, frequently leading to a disease state far more advanced and less responsive to conventional treatments than in non-smokers. Understanding the cellular and vascular alterations is key to grasping the gravity of this exposure.
it initiates a cascade of destructive biological processes that fundamentally compromise the resilience and architecture of the gum tissues
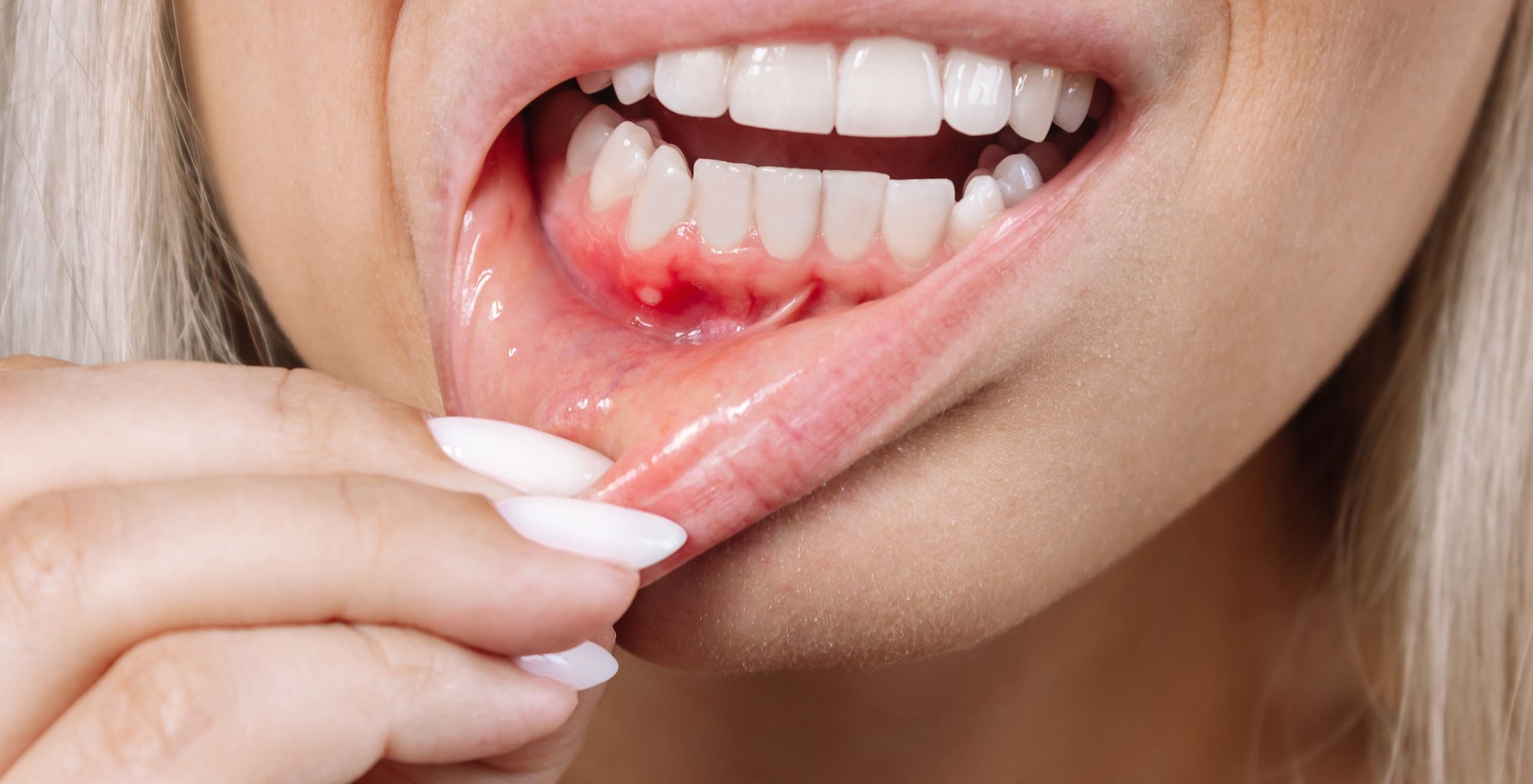
One of the most immediate and clinically misleading effects of smoking is the radical alteration it imposes upon the microvasculature of the gingiva. Nicotine, the primary addictive alkaloid, acts as a potent vasoconstrictor, immediately reducing the caliber of the minute blood vessels that supply the gum tissue. This vasoconstriction induced by smoking affects the blood supply to the Periodontal tissue, an effect that has profound implications for both disease progression and diagnosis. Reduced blood flow translates directly into diminished oxygen and nutrient delivery to the gum cells and compromised removal of metabolic waste products. Critically, this compromised blood supply suppresses a key clinical indicator of gum inflammation: bleeding. While non-smokers with gingivitis present with readily noticeable redness and bleeding upon probing—a clear warning sign—smokers often exhibit paler, more fibrotic-appearing gum tissue with significantly reduced or absent bleeding, masking the true severity of the underlying inflammatory destruction. This deceptive lack of overt clinical symptoms means the disease process is frequently allowed to advance unchecked, eroding the deeper supporting bone structure without the obvious warning signs present in non-smokers.
Furthermore, the introduction of tobacco smoke constituents directly interferes with the host’s innate and adaptive immune systems, essentially weakening the local defense against the microbial challenge that drives periodontal disease. Neutrophils, the body’s first responders in fighting bacterial infection, show impaired function in smokers, exhibiting reduced chemotaxis—their ability to migrate to the site of infection—and diminished phagocytic capacity—their ability to engulf and destroy pathogenic bacteria. The chronic exposure to smoke also influences the overall inflammatory signaling within the tissue. While inflammation is generally increased systemically, the local expression in the gums is often skewed, compromising the controlled, protective response necessary to contain the infection. This dual assault—reduced vascular supply and compromised immune surveillance—establishes a favorable environment for the proliferation of specific, more virulent bacterial species that thrive in low-oxygen conditions.
Neutrophils, the body’s first responders in fighting bacterial infection, show impaired function in smokers
The oral cavity’s resident microbial community, or oral microbiome, undergoes a dramatic and adverse transformation when repeatedly exposed to cigarette smoke. The chemical changes within the mouth, including alterations to the oxygen-reduction potential (Eh), create conditions that selectively favor the growth of anaerobic and generally more aggressive bacterial species strongly implicated in periodontitis. Studies have consistently demonstrated a distinctive microbial profile in smokers, characterized by higher levels of key periodontal pathogens such as Porphyromonas gingivalis and Treponema denticola, and a concurrent loss of beneficial or health-associated microbial species. This ecological shift is not simply a secondary event; the toxic compounds in tobacco smoke interact directly with the bacteria, disrupting the delicate equilibrium. This results in a persistent state of dysbiosis, where the microbial community itself becomes a more potent driver of inflammation and tissue destruction, significantly increasing the pathogenic load on the already compromised immune system of the host.
Beyond the immediate immune and vascular changes, smoking directly impairs the structural integrity and maintenance of the periodontal apparatus at a cellular level. Components of the smoke, particularly nicotine and carbon monoxide, interfere with the metabolism and function of critical resident cells, most notably gingival fibroblasts and periodontal ligament cells, which are responsible for producing and maintaining the collagen fibers that anchor the tooth to the bone. Nicotine has been shown to reduce collagen synthesis and increase the breakdown of existing connective tissue, an imbalance that accelerates the loss of the essential support structures. The resulting fibrous and irreversible damage to gum tissues contributes directly to increased gingival recession and the formation of deeper periodontal pockets, which in turn become inaccessible reservoirs for bacterial growth and further disease progression. This is a crucial molecular mechanism where the reparative capacity of the tissue is fundamentally undermined, ensuring that the destruction outpaces any natural attempt at repair.
fibrous and irreversible damage to gum tissues
The most devastating long-term consequence of smoking on gum health is the accelerated and disproportionate destruction of the alveolar bone, the dense structure that cradles the tooth roots. Periodontitis in smokers is characterized by greater bone loss and deeper periodontal pockets compared to non-smokers, an effect that is often dose-dependent with the number of cigarettes consumed. The underlying mechanism involves a complex interplay between increased inflammatory mediators and altered bone cell activity. Tobacco components skew the delicate balance between osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells), often promoting excessive osteoclast activity while simultaneously inhibiting the differentiation and function of osteoblasts. This unchecked resorption of bone leads to a progressive loss of tooth support, eventually resulting in tooth mobility and, ultimately, the need for extraction. The structural damage inflicted here is largely irreversible and forms the basis for the advanced, grade-D periodontitis often observed in chronic smokers.
The negative influence of smoking extends aggressively into the clinical management of periodontal disease, substantially compromising the efficacy of both non-surgical and surgical interventions. The impaired blood flow and suppressed immune response mean that healing following procedures like scaling and root planing or even advanced flap surgery is markedly diminished. Smokers consistently demonstrate a poorer clinical response, often showing less improvement in probing depth and attachment levels compared to non-smokers following treatment. For non-surgical therapy, improvements in pocket depth are often halved. In surgical contexts, especially regenerative procedures that rely heavily on robust blood supply and organized tissue repair, the failure rates are significantly higher in active smokers. This reduced therapeutic predictability creates a frustrating cycle for both patient and clinician, underscoring that cessation is a prerequisite, not just an adjunct, for successful long-term outcomes. The chronic, low-grade inflammatory state and persistent dysbiosis make the tissues inherently resistant to resolution, demanding a more intensive and less forgiving maintenance regimen.
less improvement in probing depth and attachment levels
Beyond the direct biological pathways, the behavioral factors associated with smoking interact with the biological vulnerability to further increase risk. Smokers often exhibit higher levels of calculus (tartar) accumulation, which is a hardened form of plaque that provides a rough, ideal surface for bacterial colonization and a potent mechanical irritant to the gum line. While smoking itself may not directly accelerate the rate of initial plaque accumulation, the combination of altered salivary composition, reduced dexterity in oral hygiene for some individuals, and the fundamentally compromised immune response creates a synergistic environment for disease escalation. The simple presence of higher levels of calculus (tartar) and deeper pockets creates a perpetual challenge to effective home care and professional maintenance. The physical and chemical presence of tobacco residue exacerbates the problem, demanding a higher standard of oral hygiene that the biologically compromised tissues are less able to support.
The long-term prognosis for maintaining teeth is drastically altered by smoking status. The cumulative loss of alveolar bone, coupled with the systemic resistance to effective healing, places smokers at a significantly increased risk of premature tooth loss. Furthermore, for patients who require dental implants to replace lost teeth, smoking is a recognized and significant risk factor for implant failure. The same mechanisms that impair gum and bone healing—vasoconstriction and immune suppression—directly interfere with osseointegration, the critical biological process where the titanium implant fuses with the surrounding bone. This demonstrates that the destructive footprint of smoking is not limited to the natural periodontium but extends to all tissues reliant on healthy vascularity and immune function for integration and repair. The profound effect on microcirculation ultimately defines the fate of the entire dental apparatus.
higher levels of calculus (tartar) and deeper pockets
The cellular damage observed in the oral mucosa of smokers is extensive, involving structural changes visible at a microscopic level. Histological examinations of clinically ‘healthy’ gum tissue from smokers often reveal basal cell hyperplasia, increased thickness, and signs of chronic cellular stress, including increased melanin pigmentation. This highlights that the destructive process is active even before overt clinical signs of periodontitis manifest. At the molecular level, there is a complex modulation of various inflammatory markers and cytokines, where certain pro-inflammatory pathways are stimulated while others, essential for repair, are inhibited. This internal, subclinical war ensures that the tissue is in a state of perpetual vulnerability, constantly reacting to the chemical trauma of the smoke, which is far removed from the controlled, homeostatic environment of a non-smoker’s mouth. This constant reaction to the chemical trauma of the smoke creates the foundation for eventual, irreversible tissue failure.
constant reaction to the chemical trauma of the smoke
The overall picture painted by cellular biology and clinical outcomes is one of systematic destruction, where the tobacco smoke operates as a multi-modal antagonist. It doesn’t just introduce bacteria; it blinds the immune system, chokes the blood supply, and sabotages the repair crew. The net result is a progressive, accelerated loss of the teeth’s foundational support, often occurring silently beneath a deceivingly pale and seemingly less inflamed gum line. The ultimate success in managing a smoker’s periodontal health rests not in advanced clinical techniques, but in addressing the primary systemic and local compromise—the ongoing exposure to tobacco’s volatile chemical cocktail.
