The connection between seemingly disparate health conditions often reveals a profound truth about the human body’s interconnected systems. One such relationship, increasingly recognized and studied by medical professionals, is the significant link between osteoporosis, a systemic skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue, and periodontitis, a chronic inflammatory disease that destroys the soft tissue and bone supporting the teeth. Far from being isolated incidents, the shared risk factors, underlying biological pathways, and systemic inflammatory responses suggest that what affects the strength of your hips and spine may also be playing a crucial, detrimental role in the stability of your teeth and the health of your gums. This is not simply a matter of coincidence; it points toward a unified biological mechanism where generalized bone loss finds a particularly visible, and locally destructive, outlet within the oral cavity. Understanding this bidirectional influence is paramount not only for comprehensive patient care but also for developing more integrated diagnostic and therapeutic strategies that address both the systemic and localized manifestations of skeletal and inflammatory decline.
Shared Systemic Underpinnings: Unveiling the Common Threads
The shared risk factors, underlying biological pathways, and systemic inflammatory responses suggest that what affects the strength of your hips and spine may also be playing a crucial, detrimental role in the stability of your teeth and the health of your gums.
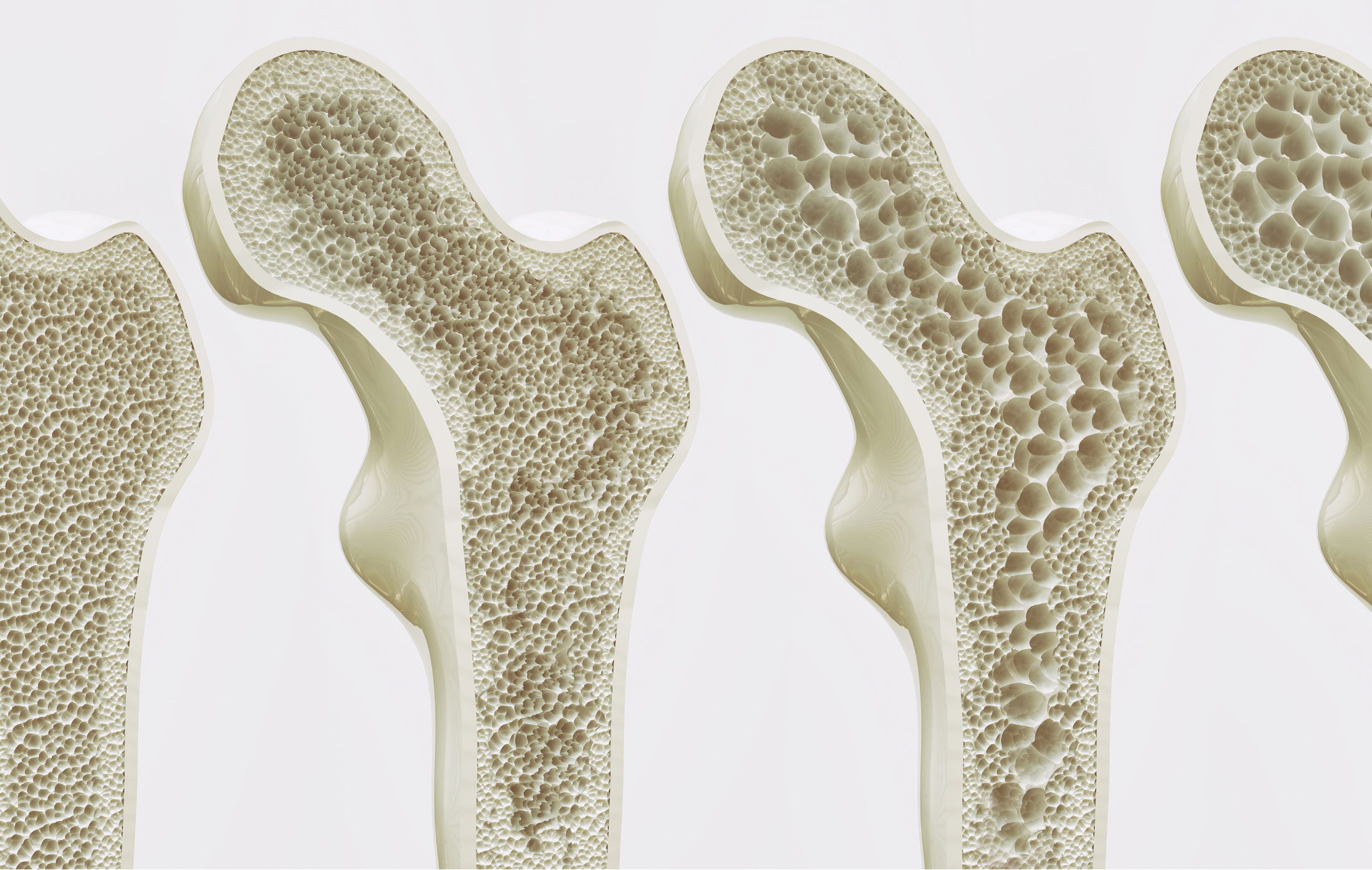
The overlap in the demographics and risk profiles for both osteoporosis and periodontitis is striking and cannot be ignored. Both conditions disproportionately affect older populations, particularly postmenopausal women, suggesting a strong hormonal component, most notably the decline in estrogen levels. Estrogen plays a vital role in maintaining the balance between bone formation by osteoblasts and bone resorption by osteoclasts throughout the skeleton. The deficiency that follows menopause accelerates the rate of bone loss, not only in the vertebrae and long bones but also in the alveolar bone, the specialized bone structure that holds the teeth in place. Furthermore, lifestyle factors such as smoking, which severely compromises bone healing and increases inflammatory burden, and poor nutrition, particularly inadequate calcium and vitamin D intake, contribute significantly to the progression of both diseases. Genetic predispositions, while complex and still being fully elucidated, also appear to contribute to an individual’s susceptibility to both rapid skeletal demineralization and aggressive gum tissue destruction. The confluence of these systemic factors creates a fertile ground where a decline in bone quality in one part of the body mirrors, or even accelerates, a similar process in the jawbone, making the mouth a potential early warning system for skeletal issues.
Hormonal Dynamics and Bone Turnover in Both Contexts
The deficiency that follows menopause accelerates the rate of bone loss, not only in the vertebrae and long bones but also in the alveolar bone, the specialized bone structure that holds the teeth in place.
The mechanism of bone turnover is central to the connection. In osteoporosis, the balance shifts in favor of bone resorption; the activity of osteoclasts outpaces that of osteoblasts, leading to a net loss of bone mass and a compromised structural integrity. This very same mechanism is active in periodontitis. The chronic presence of bacterial plaque triggers a localized immune response in the gums, leading to inflammation (gingivitis). As the disease progresses to periodontitis, the inflammatory mediators—such as prostaglandins and various cytokines like Interleukin-1$\beta$ (IL-1$\beta$) and Tumor Necrosis Factor-α (TNF-α)—activate osteoclasts in the adjacent alveolar bone. This local destruction of the alveolar ridge directly leads to the formation of periodontal pockets, gum recession, and ultimately, tooth mobility and loss. Critically, in an individual with systemic osteoporosis, the alveolar bone is already weakened due to the generalized reduction in bone mineral density. The concurrent systemic condition thus exacerbates the local inflammatory attack, accelerating the rate of bone loss around the teeth. This dual assault—systemic fragility meeting localized aggressive inflammation—creates a particularly destructive synergy. The diminished systemic bone reserve provides less resilience against the local inflammatory insult, demonstrating how hormonal shifts can predispose the entire skeleton, including the jaw, to breakdown.
The Role of Systemic Inflammation as a Bridge
The concurrent systemic condition thus exacerbates the local inflammatory attack, accelerating the rate of bone loss around the teeth.
Chronic, low-grade systemic inflammation is now recognized as a key driver and common feature of numerous chronic diseases, including both osteoporosis and periodontitis. Periodontitis is not merely a localized oral infection; the continuous presence of pathogenic bacteria and the subsequent immune response lead to the spillage of inflammatory mediators and bacterial byproducts into the bloodstream. This chronic dissemination contributes to the systemic inflammatory load. Elevated circulating levels of pro-inflammatory cytokines, those same molecules responsible for the localized bone destruction in the jaw, have been consistently linked to increased bone resorption rates and reduced bone formation across the entire skeleton. In essence, a severe, untreated case of periodontitis can fuel the inflammatory environment that favors bone loss in the spine and hips, contributing to the progression of osteoporosis. Furthermore, the systemic inflammation associated with osteoporosis itself can prime the immune system to overreact to the presence of oral bacteria, thereby increasing the severity of the periodontal breakdown. This is a complex feedback loop: periodontitis exacerbates systemic inflammation, which in turn accelerates osteoporosis, which then diminishes the bone’s capacity to withstand the local damage caused by periodontitis.
Diagnostic Challenges and Interdisciplinary Care Needs
The interpretation of routine dental radiographs can be particularly enlightening for identifying individuals who might be at an elevated risk for systemic skeletal issues.
The subtle presentation of both conditions, especially in their early stages, presents considerable diagnostic challenges. Osteoporosis is often called a “silent disease” because bone loss occurs without symptoms until a fracture occurs. Similarly, periodontitis can progress painlessly until the advanced stages. This necessitates a heightened awareness among both dental and medical practitioners. The interpretation of routine dental radiographs can be particularly enlightening for identifying individuals who might be at an elevated risk for systemic skeletal issues. Observable reductions in the height and density of the alveolar crest, changes in the mandibular cortical index (the thickness and quality of the lower jaw’s outer layer), or an increased visibility of bone trabeculae on a panoramic X-ray can serve as early, non-invasive indicators of generalized reduced bone mineral density. Dentists, by recognizing these radiographic signs, are uniquely positioned to refer patients for a Dual-Energy X-ray Absorptiometry (DXA) scan, the gold standard for diagnosing osteoporosis. Conversely, physicians treating osteoporosis should routinely inquire about and encourage thorough dental check-ups, as managing periodontal disease can potentially reduce the systemic inflammatory burden that contributes to skeletal demineralization.
Therapeutic Overlap: Leveraging Shared Mechanisms for Treatment
This provides an important avenue for coordinated care where the management of one condition can significantly benefit the prognosis of the other.
The recognition of shared biological mechanisms suggests that therapeutic interventions aimed at one condition may offer benefits for the other. Several medications used to treat osteoporosis, particularly bisphosphonates, are designed to inhibit osteoclast activity and reduce bone resorption. While primarily focused on reducing the risk of fracture in the spine and hip, these agents also theoretically slow the rate of alveolar bone loss in periodontitis. However, the use of antiresorptive drugs in patients with pre-existing periodontal disease requires careful consideration due to the rare but serious risk of medication-related osteonecrosis of the jaw (MRONJ), highlighting the need for detailed interdisciplinary communication before and during treatment. Furthermore, lifestyle modifications that are beneficial for osteoporosis, such as regular weight-bearing exercise, cessation of smoking, and adequate calcium and vitamin D supplementation, also contribute to a healthier systemic profile that aids in the control of periodontal inflammation and supports tissue repair. This provides an important avenue for coordinated care where the management of one condition can significantly benefit the prognosis of the other. The anti-inflammatory effects of certain supplements or medications could also prove dually beneficial, addressing the root cause of bone degradation in both sites.
The Critical Role of Oral Hygiene in Systemic Bone Health
The meticulous and consistent removal of pathogenic dental plaque thus becomes not just a matter of maintaining a pleasant smile, but a fundamental strategy for managing systemic inflammation and preserving overall skeletal integrity.
Given that periodontitis is initiated by bacterial plaque, the most immediate and impactful intervention lies in meticulous oral hygiene. Effective brushing, flossing, and regular professional dental cleanings and periodontal therapy are essential for reducing the local inflammatory trigger. By controlling the bacterial load and the ensuing localized immune response, the release of pro-inflammatory cytokines into the systemic circulation is minimized. This reduction in the chronic inflammatory burden may indirectly benefit systemic bone health, slowing the rate of osteoporosis progression. The meticulous and consistent removal of pathogenic dental plaque thus becomes not just a matter of maintaining a pleasant smile, but a fundamental strategy for managing systemic inflammation and preserving overall skeletal integrity. Patients with diagnosed osteoporosis should be particularly motivated to adhere to stringent oral hygiene protocols, recognizing that their skeletal fragility makes them more susceptible to the rapid and aggressive consequences of untreated gum disease. Education on this specific link can significantly enhance patient compliance and foster a more holistic approach to personal health maintenance, moving beyond the traditional separation of oral and systemic health concerns.
Future Research Directions: Beyond the Initial Correlation
Future research efforts are increasingly focused on identifying specific genetic markers and molecular pathways that predispose individuals to the simultaneous presentation of these two conditions.
While the correlation is well-established, future research efforts are increasingly focused on identifying specific genetic markers and molecular pathways that predispose individuals to the simultaneous presentation of these two conditions. Investigating polymorphisms in genes related to Vitamin D receptors, collagen synthesis, and various inflammatory cytokine production could help personalize risk assessment and preventive strategies. Furthermore, studies on the oral microbiome are exploring whether specific periodontal pathogens possess an enhanced capacity to contribute to systemic inflammation and bone loss compared to others. The development of advanced, high-resolution imaging techniques will also improve the ability to quantitatively assess subtle, early changes in alveolar bone density and structure before overt clinical symptoms of periodontitis or systemic osteoporosis manifest. Another promising area involves exploring the efficacy of targeted anti-inflammatory agents or novel growth factors that can simultaneously promote bone formation in both the generalized skeleton and the localized periodontal tissues, offering truly integrated therapeutic solutions that target the core biological dysregulation at the heart of both diseases. This move from correlation to causation, through detailed molecular and genetic mapping, is the next crucial step.
Implications for Public Health and Patient Education
The clear biological convergence necessitates a public health message that emphasizes the mouth as an integral part of the overall skeletal and vascular system, not a separate entity.
The strong evidence linking osteoporosis and periodontitis holds significant implications for public health policy and patient education. The clear biological convergence necessitates a public health message that emphasizes the mouth as an integral part of the overall skeletal and vascular system, not a separate entity. Educational campaigns should target high-risk groups, particularly postmenopausal women and smokers, clearly articulating the mutual risks. Integrating screening for periodontal disease into routine medical check-ups for individuals at risk for or diagnosed with osteoporosis, and vice versa, can lead to earlier diagnosis and intervention for both conditions, ultimately improving the quality of life and reducing the considerable morbidity and healthcare costs associated with advanced disease stages. Encouraging a seamless referral pattern between endocrinologists, gynecologists, rheumatologists, and dentists/periodontists is key to implementing truly holistic patient management. Breaking down the traditional silos in healthcare delivery is essential for leveraging this powerful link for preventative and therapeutic gains, ensuring that bone health is monitored from the jawbone to the hip.
The Significance of Alveolar Bone Density
The bone surrounding the teeth is in a state of perpetual remodeling, directly influenced by both the mechanical stresses of chewing and the continuous challenge posed by the bacterial biofilm.
The alveolar bone’s unique nature makes it a crucial junction in this disease link. Unlike the dense cortical bone of the long bones, the alveolar bone is primarily trabecular (spongy), making it metabolically more active and inherently more sensitive to changes in systemic factors like hormone levels and inflammatory mediators. Furthermore, the bone surrounding the teeth is in a state of perpetual remodeling, directly influenced by both the mechanical stresses of chewing and the continuous challenge posed by the bacterial biofilm. This dual environmental pressure—systemic fragility combined with local pathogenic assault—makes the alveolar bone a particularly vulnerable target. Any reduction in systemic bone mineral density means the alveolar bone has less reserve and resilience to withstand the localized osteoclast-activating factors released during periodontitis. Its rapid responsiveness to systemic changes is precisely why its radiographic assessment can offer invaluable early clues about the patient’s overall skeletal status, making the dentist’s role in the early detection pathway more significant than previously appreciated in generalized medicine.
A Unified Perspective on Skeletal and Oral Health
The acknowledgment of this interconnectedness fundamentally changes the diagnostic and treatment paradigm from one of isolated conditions to a model of integrated systemic health.
The journey to fully map and understand the relationship between osteoporosis and periodontitis underscores a broader shift in modern medicine: a move toward a truly unified, systemic perspective on health and disease. These two conditions are not merely comorbidities; they are manifestations of shared, underlying biological dysregulation, driven by hormonal shifts, genetic predispositions, and the common accelerator of chronic inflammation. The acknowledgment of this interconnectedness fundamentally changes the diagnostic and treatment paradigm from one of isolated conditions to a model of integrated systemic health. Moving forward, the most effective strategies will involve not just treating the fracture or arresting the gum disease, but addressing the systemic drivers—the chronic inflammation, the hormonal imbalances, and the nutritional deficiencies—that predispose the entire skeletal structure to breakdown. This integrated approach promises not only better dental outcomes but also a reduced risk of devastating osteoporotic fractures, ultimately fostering a more robust and resilient skeletal system, from the jaw to the feet.
